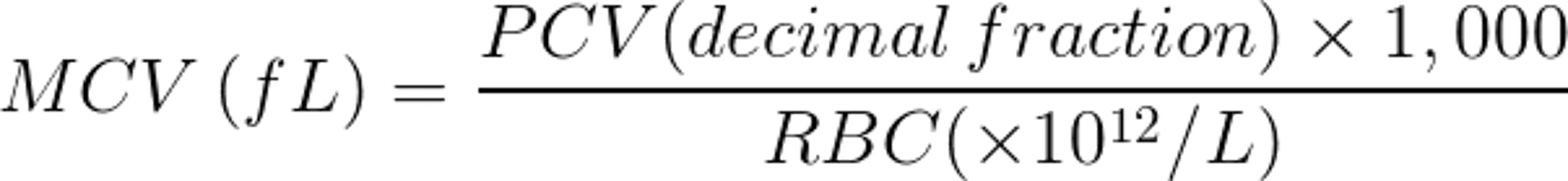Hematology refers to the study of the numbers and morphology of the cellular elements of the blood—the RBCs (erythrocytes), WBCs (leukocytes), and platelets (thrombocytes)—and the use of these results in the diagnosis and monitoring of disease. (Also see Hematopoietic System Introduction.)
Red Blood Cells:
Three RBC measurements are routinely done: packed cell volume (PCV), the proportion of whole blood volume occupied by RBCs; hemoglobin (Hgb) concentration of whole lysed blood; and RBC count, the number of RBCs per unit volume of whole blood. Although these are separate estimations, they are in effect three ways to measure the same thing, and it is incorrect to attempt to interpret them as separate variables. Inasmuch as they do vary in relation to each other, they allow calculation of two further meaningful parameters, mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC).
MCV varies widely between mammalian species, from ~15 fL in goats to ~90 fL in people. Avian and reptilian red cells are even larger, up to 300 fL. Nevertheless, MCHC varies little with species (or erythrocyte size), at ~330 g/L.
Several artifacts can cause significant and potentially misleading alterations to measured RBC parameters: 1) old samples cause RBCs to swell, thus increasing PCV and MCV and decreasing MCHC; 2) lipemia causes a falsely high Hgb reading, and hence a falsely high MCHC; 3) hemolysis causes PCV to decrease while Hgb remains unchanged, again leading to a falsely high MCHC; 4) underfilling of the tube causes RBCs to shrink, causing PCV and MCV to decrease and MCHC to increase; 5) autoagglutination causes a falsely low RBC count, and hence a falsely high MCV.
Visual description of RBC morphology on a Romanowsky stain also provides useful diagnostic information. The most common terms include 1) normocytic—cells are of normal size; 2) macrocytes—abnormally large cells, usually polychromatophilic; 3) microcytes—abnormally small cells, usually caused by a lack of hemoglobin precursors; 4) anisocytosis—variation in size of cells due to macrocytes, microcytes, or both; 5) normochromic—cells are of normal color; 6) polychromasia—variation in color of the cells, which usually describes the appearance of large, juvenile, bluish-staining polychromatophilic macrocytes (these broadly correspond to the “reticulocyte” seen with new methylene blue staining, in which the reticulum represents the remnants of the nucleus); 7) hypochromasia—decrease in staining density of the cells, usually due to a lack of hemoglobin precursors, especially iron; and 8) annulocyte—extreme form of hypochromic cell with only a thin rim of hemoglobin.
PCV is the variable usually used to assess the basic status of the erythron—increased in polycythemia, decreased in anemia—although if a sample is too hemolyzed to allow measurement of PCV, a meaningful Hgb measurement may still be obtained. RBC count as such should not be interpreted clinically.
An abnormally high PCV (polycythemia) may be relative, due to a change in the proportion of circulating RBCs to blood plasma without any change in the size of the erythron, or absolute, due to a real increase in erythron size. Absolute polycythemia may be primary (eg, polycythemia vera or, rarely, erythropoietin-producing tumors) or secondary (a consequence of disease in another organ system). (Also see Erythrocytosis (Polycythemia).)
Polycythemia vera and erythropoietin-producing tumors should be suspected only when PCV is very high, normally >0.7. The former is characterized by normal, mature RBCs and a normal (or low) erythropoietin concentration, whereas the latter may show a regenerative RBC picture with high erythropoietin concentration. Relative polycythemia may also be associated with very high PCV values, and normal, mature RBCs. Secondary polycythemia generally shows a more modest increase in PCV, often with evidence of regeneration (more so when the cause is pulmonary or cardiac, less so when the cause is hormonal). It is often possible to make the differential diagnosis of polycythemia on clinical grounds.
Abnormally low PCV (anemia) may be caused by loss of blood (hemorrhage), breakdown of RBCs in circulation (hemolysis), or lack of production of RBCs by the bone marrow (hypoplasia or aplasia). Presentation varies according to whether the condition is acute or chronic. Aplastic anemia is always chronic in onset, because anemia occurs gradually as existing cells reach the end of their lifespan. (Also see Anemia.)
In acute hemorrhagic anemia, external blood loss is easily appreciated clinically, but blood loss into a body cavity may be determined only on paracentesis. Initially, all hematologic parameters may be normal, because it may take 12 hr for fluid shifts to produce a decrease in the PCV. Within a few days, RBCs become regenerative, with juvenile forms appearing in circulation (except in horses, in which circulating evidence of regeneration is not readily appreciable). These consist of polychromatophilic macrocytes and normoblasts (nucleated RBCs). Late normoblasts have a small, nonviable nucleus and a moderate amount of cytoplasm colored similarly to that of the polychromatophilic macrocytes, whereas early normoblasts have a larger, viable nucleus and scanty cytoplasm. These are most easily distinguished from lymphocytes by their more densely staining nucleus.
If substantial amounts of blood have been lost from the body, the RBC picture may become hypochromic. Thus, this type of anemia shows an increase in MCV and a decrease in MCHC. If bleeding is into a body cavity, hypochromasia may not be evident because hemoglobin precursors will be recycled. However, slight jaundice may be seen as the sequestered cells are broken down. Some sequestered cells may also be returned to the circulation intact, if somewhat misshapen.
In acute hemolytic anemia, PCV will decrease immediately, and in the early stages some jaundice will be evident. In the very early stages, even a sample collected with extreme care may be markedly hemolyzed. As with hemorrhagic anemia, the RBCs will become regenerative within a few days, with polychromatophilic macrocytes and nucleated RBCs evident. Because hemoglobin precursors are not lost from the body, true hypochromasia is not seen.
Chronic hemorrhagic anemia may be difficult to appreciate if blood is lost in the feces or urine, or due to bloodsucking ectoparasites. Anemia may be severe, and the RBC picture will be regenerative on presentation. Hypochromasia is usually very marked. In very longstanding conditions, depletion of iron and other hemoglobin precursors can become so marked that most of the cells are microcytic, and MCV may paradoxically decrease. Intermittent intra-abdominal hemorrhage leads to a somewhat different picture, because blood shed into the peritoneal cavity can be returned to the circulation. PCV may therefore recover quickly (until the next episode), and signs of depletion of hemoglobin constituents do not emerge.
In chronic hemolytic anemia, RBCs are regenerative on presentation, except that some cases of autoimmune hemolytic anemia (AIHA) paradoxically show little or no regeneration until treatment has been initiated. Hypochromasia is less marked than in hemorrhagic conditions, and misshapen RBCs (including target cells and folded cells) are more common. The spherocyte, in which the erythrocyte loses its classic biconcave shape, is essentially pathognomonic for AIHA. Jaundice may be absent, because the products of the destruction of the RBCs may be cleared by the reticuloendothelial system and the liver as quickly as they are formed.
Courtesy of Dr. John W. Harvey.
Courtesy of Dr. John W. Harvey.
Hypoplastic and aplastic anemia may be mild if RBC production is merely depressed secondary to some other disease. Protein, mineral, or vitamin deficiencies may cause hypoplastic anemia, but these are more likely to be secondary to another disease (eg, chronic hemorrhage or malabsorption) than simple dietary deficiency. Other diseases may cause depression of erythropoietin production, eg, renal failure, deficiencies of hormones that usually stimulate erythropoietin production (eg, hypothyroidism, hyperadrenocorticism), and chronic, debilitating conditions (eg, chronic infections, chronic parasitism, and neoplasia). RBC morphology is nonregenerative and may be hypochromic if a deficiency state is involved. Paradoxically, vitamin B12 and/or folic acid deficiency produces a macrocytic RBC picture due to early maturation arrest of the erythrocytes. Neoplasia of the bone marrow may cause severe anemia as erythropoietic elements are crowded out, but some regeneration may be seen as the remaining bone marrow attempts to compensate. In this case, other bone marrow cell lines will also be affected.
Courtesy of Dr. John W. Harvey.
True aplastic anemia refers to a failure of the entire bone marrow. The shorter-lived granulocytes and platelets decrease first, followed by a progressively severe anemia that is normocytic and normochromic.
White Blood Cells:
The WBCs consist of the granulocytes (neutrophils in most mammals, called heterophils in rabbits, reptiles, and birds [and these look like eosinophils in smears]; eosinophils; and basophils) and the agranulocytes (lymphocytes and monocytes). Although each type is traditionally counted by determination of its percentage of the total WBC population, meaningful interpretation requires that the absolute number of each type be calculated by multiplying the total WBC count by the fraction attributable to the individual cell type. Percentages of each cell type alone are not helpful. An increased percentage that is due to an absolute decrease in another cell type is not an increase at all.
Mature neutrophils have a lobulated nucleus, but when demand is high, immature cells with an unlobulated band nucleus (no constriction of the nucleus is more than half the width of the nucleus) may be released into circulation. They function as phagocytes and are important in infectious conditions and in inflammation. Increased neutrophil counts (neutrophilia) are caused by inflammation, bacterial infection, acute stress, steroid effects, and neoplasia of the granulocytic cell line (granulocytic leukemia can be difficult to differentiate from a simple neutrophilia without special stains or bone marrow biopsy). Decreased neutrophil counts (neutropenia) are caused by viral infections, toxin exposure (including foodborne toxins), certain drugs (eg, carbimazole and methimazole), autoimmune destruction of neutrophils, bone marrow neoplasia not involving the granulocytes, and bone marrow aplasia.
Eosinophils are characterized by prominent pink-staining granules on a Romanowsky stain. They inactivate histamine and inhibit edema formation. Increased eosinophil counts (eosinophilia) are caused by allergic/hypersensitivity reactions, parasitism, tissue injury, mast cell tumors, estrus, and pregnancy or parturition in the bitch. Some large dog breeds (eg, German and Belgian Shepherds, Rottweilers) normally have a relatively high eosinophil count. Extremely high eosinophil counts (hypereosinophilic syndrome), possibly due to an out-of-control hypersensitivity reaction, and eosinophil leukemia (a form of chronic myeloid leukemia) are also described. Decreased eosinophil count (eosinopenia) is almost always caused by the action of glucocorticoids, either endogenous or therapeutic.
Basophils are rare in most species and are characterized by blue-staining granules on a Romanowsky stain. They are more easily seen in cattle. They are closely related to mast cells and, like them, initiate the inflammatory response by releasing histamine. An increased basophil count (basophilia) accompanies eosinophilia in some species as part of the hypersensitivity reaction.
Monocytes are large cells with blue-gray cytoplasm, which may be vacuolated, and a kidney bean-shaped or lobulated nucleus. Their main function is phagocytosis, and they are essentially identical to tissue macrophages. An increased monocyte count (monocytosis) may occur in any chronic disease, especially chronic inflammation, and may be very marked in neoplasia. Monocytes also increase as part of the steroid response in dogs.
Lymphocytes mainly develop outside the bone marrow in the lymph nodes, spleen, and gut-associated lymphoid tissue. They are the smallest of the WBCs, with a round, evenly staining nucleus and sparse cytoplasm. Larger, reactive lymphocytes can be seen after antigenic stimulation, and care must be taken to differentiate them from neoplastic lymphocytes. Their primary function is immunologic, including both antibody production and cell-mediated immune responses. Some survive only a few days, but many are long-lived. The number in circulation is a balance between populations in the blood, lymph, lymph nodes, and splenic follicles and does not necessarily reflect changes in lymphopoiesis. An increased lymphocyte count (lymphocytosis) may occur for physiologic reasons, especially in cats, but significant increases usually indicate leukemia. Immature or bizarre cells may also be recognized. Decreased lymphocyte counts (lymphopenia) are usually due to an effect of corticosteroids, either endogenous (stress or Cushing disease) or therapeutic, and may also accompany neutropenia in some viral infections, especially the parvoviruses. Lymphopenia may also be a feature of solid-organ lymphosarcomas, when leukemia is absent.
Platelets:
Courtesy of Ontario Veterinary College.
Courtesy of Ontario Veterinary College.
Courtesy of Ontario Veterinary College.
Mammalian platelets are pale blue granular fragments (much smaller than RBCs) shed from multinucleate megakaryocytes in the bone marrow; avian and reptilian platelets are true cells with nuclei. They maintain the integrity of the endothelium and act as part of the clotting process to repair damaged endothelium, where they ensure mechanical strength of the clot.
Increased platelet counts (thrombocytosis) occur as a reaction to consumption after injury, when large juvenile platelets may also appear; after splenectomy, as splenic stores are liberated to the circulation; after vincristine treatment, which increases platelet shedding from megakaryocytes; and in megakaryocytic leukemia.
Decreased platelet counts (thrombocytopenia) are caused by autoimmune reactions, thrombotic/thrombocytopenic purpura, bone marrow suppression and aplasia, bone marrow neoplasia, and equine infectious anemia. Signs are petechiation and ecchymosis more than frank hemorrhage, and little may be seen until the platelet count is < 20 × 109/L. Platelet functional abnormalities present similarly, but platelet numbers and morphology are normal.
Blood Sample Preparation and Evaluation:
In-house hematologic investigations, with a minimum of specialist equipment, can provide almost as much information as a full laboratory analysis, although some estimations are qualitative rather than quantitative.
Blood for hematology should be collected into tubes containing EDTA anticoagulant and immediately mixed well to avoid clotting. Larger (2.5 mL) tubes yield better results than the smaller (1 mL) pediatric tubes and are less likely to clot. Nevertheless, it is essential to fill the tube exactly to the mark, so smaller tubes may be unavoidable for small patients. WBC morphology deteriorates most quickly, especially in equine blood, so if the sample cannot be analyzed immediately, a thin blood smear should be submitted to the laboratory with the blood sample.
PCV is measured by microhematocrit, which is the reference method. A capillary tube is filled ¾ full with well-mixed blood and sealed at one end; heat-sealing is best if a Bunsen burner is available, otherwise a proprietary clay pad is used. The tube is spun in a high-speed microhematocrit centrifuge for 6 min, and the PCV is read using a microhematocrit reader with a sliding cursor. The appearance of the plasma (eg, normal, icteric, hemolyzed, lipemic) and the thickness of the buffy coat, which gives a very rough guide to total WBC count, should be noted.
Further information is obtained from a blood film. This is made by using one slide, with a corner broken off as a spreader, to pull a small drop of blood across a clean slide into a thin film. A suitable film is thin (one erythrocyte thick) and tapers to a feathered edge before the far end of the slide. The broken corner of the spreader slide ensures two straight edges parallel to the long edges of the slide. Immediately after the film is spread, it is dried quickly by fanning the slide in the air. Air-dried smears can be sent to the laboratory in a slide mailer or stained and examined in the practice. Commercial rapid Romanowsky-type stains merely require the slide to be dipped in three colored solutions in succession. Cell morphology is clear and comparable to more permanent stains such as Leishman or May-Gruenwald-Giemsa, although quality deteriorates after a few days. The slide should be allowed to dry naturally or dried with a hair dryer (not wiped dry), and examined under oil immersion.
Clinically useful information may be easily achieved in-house for all hematologic variables. The main deficiency is the absence of a numerical WBC count, and hence of numerical values for the differential WBC count. This may be acceptable for an interim emergency investigation, and a WBC count using a hemocytometer slide may be attempted. A mirrored slide with an Improved Neubauer ruling may be used, with a cover slip fitted so that Newton rings are visible on both sides. The blood sample is diluted 1:20 with Turck fluid or a similar diluent. An automatic pipette capable of dispensing 0.95 mL and 0.05 mL (50 μL) should be used to ensure an accurate dilution. The sample should be mixed well and allowed to stand for 10 min while the stain is taken up by the cells. The chamber of the hemocytometer is then filled by using a capillary (PCV) tube. The number of cells in each of the four large corner squares of the grid are counted, and the total divided by 20 to calculate the total WBC count × 10 9/L.
Several hematologic instruments are available for in-practice use. Those based on centrifugation, in which WBC measurements are made by spreading a stained buffy coat, are not true hematology analyzers, but give approximate counts. Although a numerical estimate of total WBC count is provided, the differential WBC count cannot distinguish between lymphocytes and monocytes, and results do not always correlate well with standard methods. This method is appropriate only as an emergency approximation, and a blood film must always be examined in addition. It is also wise to check the PCV by microhematocrit.
Impedance counters (Coulter principle) are used by diagnostic laboratories and perform well in experienced hands. However, it is difficult to achieve optimal performance without trained technical staff. Instruments not based on the Coulter principle should be avoided for veterinary use, because their results do not necessarily compare well on nonhuman samples. Instruments providing automated differential counts perform poorly on nonhuman blood, and results must never be accepted without also checking a blood film. No hematology laboratory should be without the facility for examining blood films, and a blood film should be examined for every patient sample. Quality assurance issues are the same as those for biochemistry laboratories ( see Clinical Biochemistry). If accuracy and reliability cannot be guaranteed to the same standard as the referral laboratory, then results should not be relied on without external confirmation.
Red Blood Cells:
The size, uniformity of size, and presence of microcytes, macrocytes, and abnormally shaped cells should be noted, along with cell color, uniformity of color, and the presence of hypochromasia, polychromasia, and nucleated RBCs. An overall descriptive assessment of the RBC picture should be made, including the degree of regeneration or hypochromasia, if any.
White Blood Cells:
A qualitative estimate of numbers should be made (ie, very low, low, normal, high, very high). This can be remarkably consistent with practice. The proportions of each cell type can be estimated, or preferably, a formal differential count of 100–200 cells performed. Unusual or abnormal WBC forms (eg, band or toxic neutrophils), or pathologic cells (eg, prolymphocytes, lymphoblasts, or mast cells) should be noted.
Platelets:
A qualitative estimate of platelet numbers, based on how many can be seen in a typical high-power field (oil immersion) should be made. Several fields should be examined, more if numbers appear low. Results can be ranked as none seen (on entire slide), rare (very few on entire slide), low in number (< 5 per high-power field), adequate (5–20 per high-power field), or abundant (>20 per high-power field). Normal platelet numbers in the horse are about half those of other species. In a sample more than a few hours old, platelets may clump into rafts, leaving areas of the slide apparently devoid of platelets. Slides should be scanned for rafts before reporting platelet numbers as low. Enlarged or macroplatelets should also be noted.