Courtesy of Dr. Mark D. Kittleson.
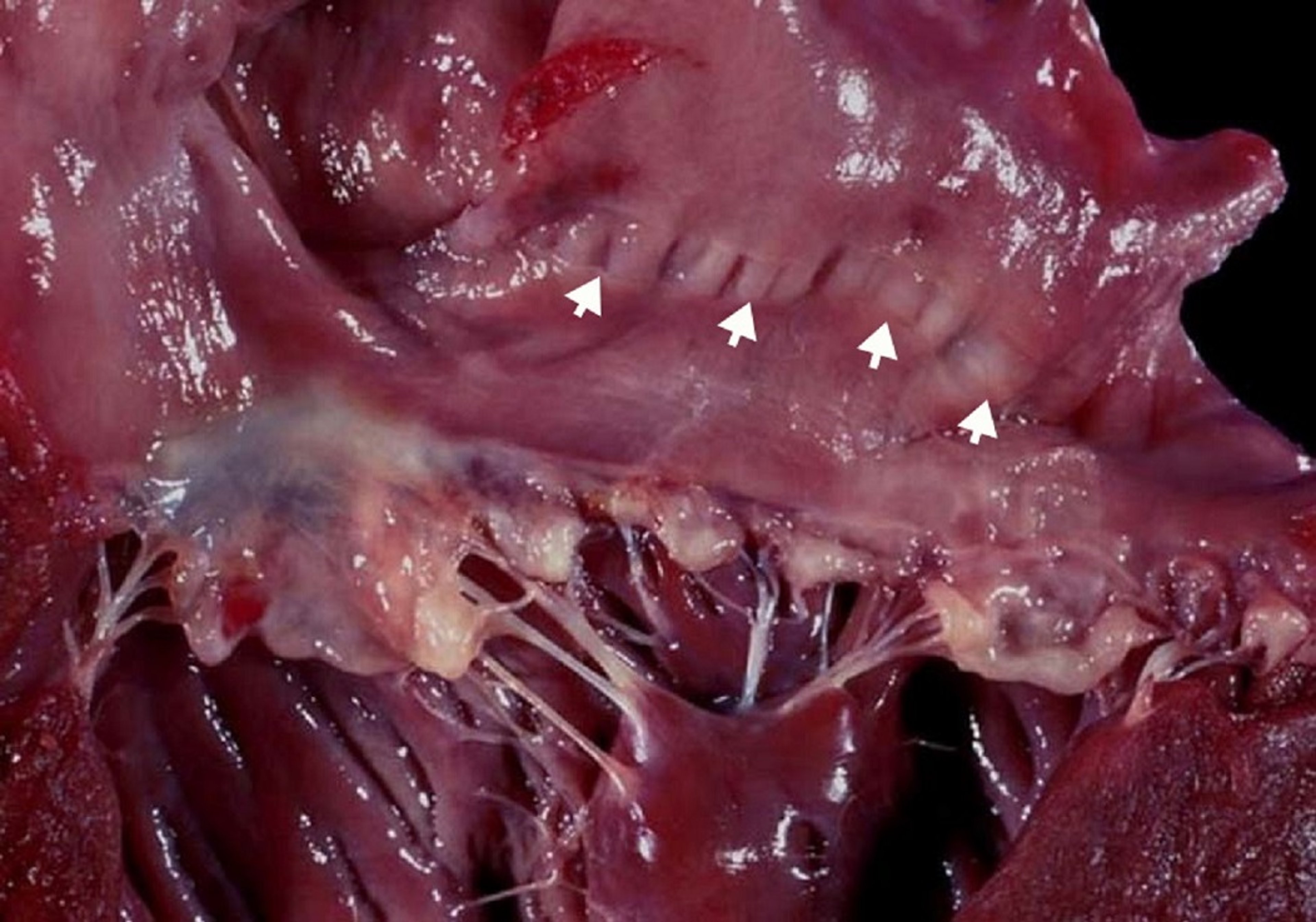
Myxomatous degeneration is a process by which the fibrous layer of an atrioventricular (AV) valve breaks down to cause valve prolapse (hooding) and the spongiform layer proliferates to cause nodular thickening of the cardiac valve leaflets, most severely at their tips. Myxomatous degeneration commonly affects the mitral and tricuspid valves in older dogs. Chordae tendineae are also affected by the degenerative process, making them prone to rupture. The exact cause of myxomatous degeneration is unknown; in Cavalier King Charles Spaniels and Dachshunds, however, it is an inherited trait. Myxomatous degenerative valve disease is the most common cardiac disease in dogs, accounting for ~75% of cardiovascular disease in this species; ~60% of affected dogs have only the mitral valve affected, 30% have lesions in both the tricuspid and mitral valves, and < 10% have only tricuspid valve disease. In dogs, the disease is age and breed related, with older, small-breed dogs demonstrating a much higher incidence. Cats can also have myxomatous AV valve degeneration (most commonly affecting the mitral valve leaflets); however, it is uncommon.
Insufficiency of an AV valve results in turbulent systolic flow through the affected valve from a ventricle into an atrium. This regurgitation results in a systolic heart murmur and an increase in volume within the atrium, and thus an increase in atrial chamber size. When regurgitation is severe, atrial pressure may also increase. If the mitral valve is affected, the increased left atrial pressure results in increased pulmonary capillary pressures and, if the increase is high enough (ie, > 20 mm Hg), in cardiogenic pulmonary edema (CHF). If the tricuspid valve is affected, severe regurgitation can result in an increased systemic venous pressure and clinical signs of right heart failure (in dogs, most commonly ascites). The constant, high-velocity, regurgitant jet of blood through the affected mitral valve physically damages the endocardium of the left atrium, resulting grossly in jet lesions. In cases with severe regurgitation, the chronic increase in left atrial size and pressure can lead to left atrial rupture and acute cardiac tamponade, often resulting in death.
Pathophysiologically, the body compensates for valvular regurgitation primarily by renal retention of sodium and water, causing an increase in blood volume and in venous return to the heart. The associated enlargement in ventricular chamber size makes the left ventricle capable of ejecting a larger total stroke volume with each beat, such that even though some percentage of blood is flowing backward into the left atrium, a normal or near-normal amount can be ejected forward into the aorta. This mechanism is highly efficient and enables the heart to compensate over chronic periods of time and also an extreme amount of regurgitation. For example, a small dog can completely compensate for regurgitation in which as much as 75% of the blood flowing from the left ventricle goes into the left atrium, while only 25% goes forward into the aorta. As a result of this compensation, only ~30% of dogs with mitral regurgitation ever develop CHF.
In dogs, there are no clinical signs in the early and middle stages of the disease, although a systolic murmur (grades 1–6) is heard with maximal intensity at the left apex. When the mitral regurgitation becomes severe and overwhelming, CHF manifests as pulmonary edema, producing tachypnea, dyspnea, and sometimes a cough. Syncope may also occur. Sudden death is rare but may occur secondary to left atrial rupture or rupture of a primary mitral valve chord.
CBC, serum chemistry profile, and urinalysis are usually within normal limits in cases of valve degeneration. Measurement of the plasma or serum concentration of N-terminal pro-B-type natriuretic peptide (NT-proBNP) may be useful in dogs with mitral regurgitation: it is usually not increased in dogs with mild mitral regurgitation, may be increased in some dogs with moderate to severe regurgitation, and is increased in most dogs with CHF secondary to mitral regurgitation. Left atrial enlargement is the characteristic finding on thoracic radiographs of an animal with degeneration of the mitral valve, and the size of the left atrium correlates directly with the severity of the regurgitation in small dogs. The exception to this rule is that when acute heart failure is due to ruptured chordae tendineae, the left atrium is not severely enlarged.
Echocardiography demonstrates thickened and irregular valve leaflets of normal to increased echogenicity in cases of valve degeneration. Chordae tendineae may be ruptured, causing the AV leaflets to flail (ie, the leaflet tips to protrude) into the atrium during ventricular contraction. Color flow Doppler echocardiography can be used to document the presence of mitral regurgitation. The size of the color flow jet alone or in comparison to the size of the left atrium (ie, ratio of the jet to the left atrial area) should not be used to assess the severity of the mitral regurgitation. In general, the size of the left atrium is a more reliable estimate of severity.
On ECGs, animals with mild to moderate degenerative valve disease show a normal sinus arrhythmia or normal sinus rhythm. When congestive heart failure (CHF) develops, the increase in sympathetic tone often results in the loss of sinus arrhythmia and usually in an increase in heart rate (ie, sinus tachycardia). Left atrial enlargement promotes the development of atrial arrhythmias such as atrial premature complexes and atrial fibrillation. Ventricular tachyarrhythmias are uncommon.
Surgical treatment of mitral regurgitation is routine in human medicine and most commonly consists of mitral valve repair. Successful mitral valve repair has also been accomplished in dogs; currently, however, the only successful program is in Japan and is expensive (the Mighty Hearts Project provides more information). Replacement of the mitral valve with a prosthetic valve is almost uniformly unsuccessful.
Predicting which dogs with mitral regurgitation will go into heart failure is difficult. Pimobendan slows disease progression, so it should be administered once the left atrium is at least moderately enlarged. Amlodipine and hydralazine, which decrease the amount of regurgitation and improve perfusion, can be very effective in addition to diuretic therapy. In acute and severe CHF, oxygen and aggressive parenteral furosemide administration are warranted. Nitroprusside can also be beneficial. Some affected dogs can live for > 1 year with appropriate treatment. However, survival time is highly variable, and no firm estimates should be provided. If a dog has been treated for left heart failure for > 2 years, the diagnosis should be reassessed.
For More Information
ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33(3):1127-1140. doi: 10.1111/jvim.15488